Methadone Treatment for Opioid Use Disorder
Methadone Treatment for Opioid Use Disorder
Methadone Treatment for Opioid Use Disorder
To help navigate a complex evidence base, this page is a quick stop for policymakers considering MOTAA. It provides access to high quality studies of policy affecting methadone treatment for OUD, and other important materials.

- Home
- -
- Advocacy
- -
- National Advocacy
- -
- Methadone Tx for OUD Policymaker Resources Methadone Tx for OUD Policymaker Resources
Download an infographic illustrating the limited reach of methadone Tx for OUD in a range of addiction treatment programs under current federal law here.
The vast majority of non-hospital-based residential, partial hospitalization, and intensive outpatient addiction treatment programs do not have an OTP. Furthermore, OTPs, which are technically outpatient medical practices, are not required under federal regulations to involve addiction specialist physicians in a patient's care.
Download a helpful explanation to the Modernizing Opioid Treatment Access Act ("MOTAA") here.
MOTAA would authorize the Drug Enforcement Administration (DEA) to issue special registrations for physicians who are board-certified in addiction medicine and addiction psychiatry, who could then use their clinical expertise in prescribing methadone for OUD treatment that could be dispensed from community pharmacies, subject to the Substance Abuse and Mental Health Services Administration's (SAMHSA) rules or guidance on supply of methadone for unsupervised use.
Download addiction clinicians' letter to Congress here.
Lead by Dr. Ruth A. Potee, Medical Director of the Franklin County House of Corrections and Director of Addiction Services for the Behavioral Health Network, hundreds of physicians and other addiction healthcare professionals penned a letter to the Congressional Committees on Energy and Commerce (E&C), and Health, Education, Labor, and Pensions (HELP) earlier this year, urging Congress' swift passage of MOTAA.
Download stakeholders' letter to Congress supporting MOTAA here.
Over one hundred stakeholders, unified in the belief that MOTAA will help turn the tide on the nation's addiction crisis, sent a letter to leaders in Congress, the E&C Committee, and the HELP Committee, underlining the importance of the legislative fix in addressing the shortage of methadone treatment for OUD that contributes to racial, gender, and geographic inequities in access to such treatment, especially in rural areas.
Download the bill text to MOTAA here.
This federal legislation would no longer criminalize such prescribing, as described above, at the federal level, while preserving federal (and state) laws that guard against diversion of controlled medications, and remaining subject to federal restrictions regarding unsupervised supply. It also provides additional federal safeguards at the federal level, requiring electronic prescriptions dispensed only to patients, and making it logistically possible for retail pharmacists, who regularly input prescription data into prescription drug monitoring programs (PDMPs), to include methadone for OUD in PDMPs.

New Study Examines Association Between Policy Change and Methadone-Involved Overdose Deaths, Comparing States That Relaxed Take-Home Dose Restrictions With Those That Did Not
An August 16th, 2024 study did not find any evidence of a link between extended take-home methadone doses and increased overdose mortality.
The author writes:
"A criticism directed at the federal policy change is that easing restrictions might increase the risks of overdose and diversion. This study did not find any evidence of a link between extended take-home doses and increased mortality. Moreover, recent research has shown that the extended take-home policy was associated with fewer methadone-related deaths among Black and Hispanic men. A possible explanation for the decrease is the demeaning experience of having to daily report to an OTP for Black and Hispanic men, who are already marginalized and continually exposed to systems of surveillance, stigma, and punishment. The provision of additional take-home doses introduced a semblance of normalcy and dignity that was absent with regular OTP attendance. This change reduced how often patients needed to visit clinics and gave them more control over their treatment, potentially enhancing adherence and overall well-being. The extended take-home policy may have lowered certain social and economic barriers to treatment, like transportation costs and conflicts with work schedules, which disproportionately affect marginalized communities. Also, this added flexibility could have strengthened the therapeutic alliance between healthcare providers and patients by fostering trust and increasing patients’ involvement in their treatment.
Similarly, available research suggests that diversion of methadone was low among patients receiving extended take-home doses. Nevertheless, even with the strict protocols governing methadone treatment, informal sharing sometimes occurs, often to help friends or family members manage the pain of opioid withdrawal. The impact of such diversion on the illicit drug market appears minimal, given methadone’s limited psychotropic effects."

Top Government Researcher Calls For Easier Access To Drug That Treats OUD: "No Reason Why Not"
The top addiction researcher in the U.S. government has called for the broad deregulation of methadone in a major departure from how it is currently made available. Dr. Nora Volkow, who is director of the National Institute on Drug Abuse (NIDA), made her comments, as reported by STAT News, at a recent Stat Summit.
Dr. Volkow was clear in her comments about her desire for methadone to be available by prescription. "There's absolutely no reason why not [to allow prescription of methadone]," Volkow told the summit.
High-Quality Studies You Should Know

Fentanyl Found Responsible for Nearly Half of Opioid Overdose Deaths in 2020
Simulation Model Finds Fentanyl and COVID-19 Disruptions Drove Excess Opioid Overdose Deaths
Using a validated simulation model of the opioid overdose crisis called SOURCE, which can help the study of dynamic behavior in response to conditions that cannot be easily applied in real life, researchers enumerated the relative contribution of factors to the unprecedented 38% rise in opioid overdose deaths in 2020 - an estimated 18,276 potential excess deaths. Through the simulation, they found that the rise of fentanyl in the heroin supply continued to play a singular role in opioid overdose deaths, contributing to 43% of such excess deaths and highlighting the urgency of mitigating its effect on overdoses. A confluence of various COVID-19-related factors were likely responsible for the majority of other excess opioid overdose deaths.
Stringfellow, Erin J, Tse Yang Lim, Catherine DiGennaro, Zeynep Hasgul, and Mohammad S Jalali. “Enumerating Contributions of Fentanyls and Other Factors to the Unprecedented 2020 Rise in Opioid Overdose Deaths: Model-Based Analysis.” PNAS Nexus 2, no. 4 (March 3, 2023): pgad064.
Access here.
Methadone Treatment Outcomes Under Relaxed Regulations Were Non-Inferior to Treatment Outcomes the Year Before COVID-19
Six Month Retention and Rates of Adverse Events Were Similar Compared To Methadone Patients With Daily OTP Attendance the Year Earlier, Despite Higher Rates of Opioid Use
In a retrospective observational cohort examination of nine geographically dispersed opioid treatment programs (OTPs), authors used electronic health records (EHRs) to compare patients newly-enrolled between April 15th and October 14, 2020 (after OTPs were granted novel flexibilities beginning March 15, 2020 that allowed for reduced visit frequency and extended take-home doses), with patients enrolled March 15 and September 14, 2019.
A total of 821 individuals were newly admitted in the post-COVID and year-ago control periods, with an average age of 38.3 (SD 11.1) that was 58.9% male. The only difference across these two groups was the prevalence of psychostimulant use disorder (25.7% vs 32.9%, p = 0.02). In the post-COVID period, patient retention was non-inferior (60.0% vs 60.1%), as were hazards of adverse events in the aggregate (X2 (1) = 0.55, p = 0.46). However, rates of month-level opioid use were higher among post-COVID intakes compared to pre-COVID controls (64.8% vs 51.1%, p < 0.001).
The findings of this study suggest that allowing for up to 14 and 28 days of take-homes early in care may not be associated with increased dropout or adverse events, consistent with pre-COVID studies that showed no differences in retention with less frequent in-person addiction medication dosing.
Williams, Arthur Robin, Noa Krawczyk, Mei-Chen Hu, Lexa Harpel, Nicole Aydinoglo, Magdalena Cerda, John Rotrosen, and Edward V. Nunes. “Retention and Critical Outcomes among New Methadone Maintenance Patients Following Extended Take-Home Reforms: A Retrospective Observational Cohort Study.” The Lancet Regional Health – Americas 0, no. 0 (December 4, 2023). https://doi.org/10.1016/j.lana.2023.100636.
Access here.
Methadone-Involved Overdose Deaths During Pandemic Likely Driven By Unregulated Drug Market
Illicitly Made Fentanyl, Not Policy Changes, Likely Drove Such Deaths During COVID-19
Using an interrupted time-series analysis, which is increasingly used to evaluate the impact of large-scale health interventions more accurately, authors examined methadone-involved overdose deaths from January 2019 to August 2021. Methadone-involved overdose deaths were unlikely to be associated with the blanket exception to provide take-home methadone doses allowed by the Substance Abuse and Mental Health Services Administration (SAMHSA) by state request. The modest increase in methadone-involved overdoses in March 2020 was likely associated with the spike in overall drug overdose deaths driven by illicitly made fentanyl in the early months of the COVID-19 pandemic. In sum, the trends they identified are as follows:
In March 2020, overdose deaths both with and without methadone increased consistently. After March 2020, overdose deaths not involving methadone continued to increase approximately 69 deaths per month, whereas methadone-involved overdose deaths remained stable. Finally, trend slopes were similar, or the percentage of overdose deaths involving methadone declined at similar rates, approximately 0.05% to 0.06%, before and after the take-home policy change, with 4.5 % of overdose deaths involving methadone in January 2019, and declining to 3.2% by August 2021.
Jones, Christopher M., Wilson M. Compton, Beth Han, Grant Baldwin, and Nora D. Volkow. “Methadone-Involved Overdose Deaths in the US Before and After Federal Policy Changes Expanding Take-Home Methadone Doses From Opioid Treatment Programs.” JAMA Psychiatry 79, no. 9 (September 1, 2022): 932–34.
Access here.
Racial and Ethnic Disparities May Have Been Narrowed in Methadone-Involved Overdoses by Policy Changes
Take-Home Methadone Flexibilities Improving Access to Treatment May Have Saved Lives Among Black and Hispanic Men
Utilizing an interrupted time-series analysis and examining 14,529 methadone involved overdose deaths, these authors looked at the period from January 1, 2018, to June 30, 2020, and found an association between the flexibilities allowing for more methadone take-home doses to patients and reduced monthly methadone-involved overdose deaths among Black and Hispanic men. They found no association of the flexibilities with deaths of Black and Hispanic women or White men or women, however.
The authors dispute the findings of Kleinman and Sanches described below on this resource page:
"In examining racial and ethnic differences in methadone-involved mortality, Kleinman and Sanches found that the number of individuals with methadone-involved deaths increased in each group (overall by 39%) between the 12-month periods before and after March 2020, with the largest increases among Hispanic (70%) and non-Hispanic Black individuals (57%). However, when assessing trends, their year-over-year analysis did not control for the step increase in mortality level that occurred in the early spring of 2020. The authors assumed that the step increase could be attributed to the change in methadone regulations, yet the increase in level also has been found in drug overdose deaths that did not involve methadone and in related domains, such as alcohol use disorder–associated deaths. Disruptions related to COVID-19 provide a more intuitive and parsimonious explanation for the step increases."
Harris, Rebecca Arden, Judith A. Long, Yuhua Bao, and David S. Mandell. “Racial, Ethnic, and Sex Differences in Methadone-Involved Overdose Deaths Before and After the US Federal Policy Change Expanding Take-Home Methadone Doses.” JAMA Health Forum 4, no. 6 (June 9, 2023): e231235.
Access here.
Patients Prescribed Methadone May Have Been Protected From Overdose in UK
Being Prescribed Methadone May Have Been Protective of Methadone-Related Overdose
In a retrospective post-mortem toxicology study of opioid agonist therapy (OAT)-related deaths in England, which experienced a similar increase in methadone-involved overdose deaths to the U.S., researchers comparing a three-month period from March to June in years 2016 to 2020 found in a 64% increase in methadone-related deaths in 2020 compared to 2019. The authors note that the 74% increase in the mortality rate of decedents not prescribed methadone was considerable - compared to the 22% increase in the mortality rate of in-treatment decedents.
According to the authors, this indicates that being prescribed methadone may have been protective, who also note that the data indicates in the U.S. that the increase in methadone-involved deaths during March 2020 "were related to an increase in deaths primarily attributable to co-administered fentanyl."
Aldabergenov, D., L. Reynolds, J. Scott, M. J. Kelleher, J. Strang, C. S. Copeland, and N. J. Kalk. “Methadone and Buprenorphine-Related Deaths among People Prescribed and Not Prescribed Opioid Agonist Therapy during the COVID-19 Pandemic in England.” The International Journal on Drug Policy 110 (December 2022): 103877.
Access here.
States That Have Concurred With SAMHSA's Extended Flexibilities
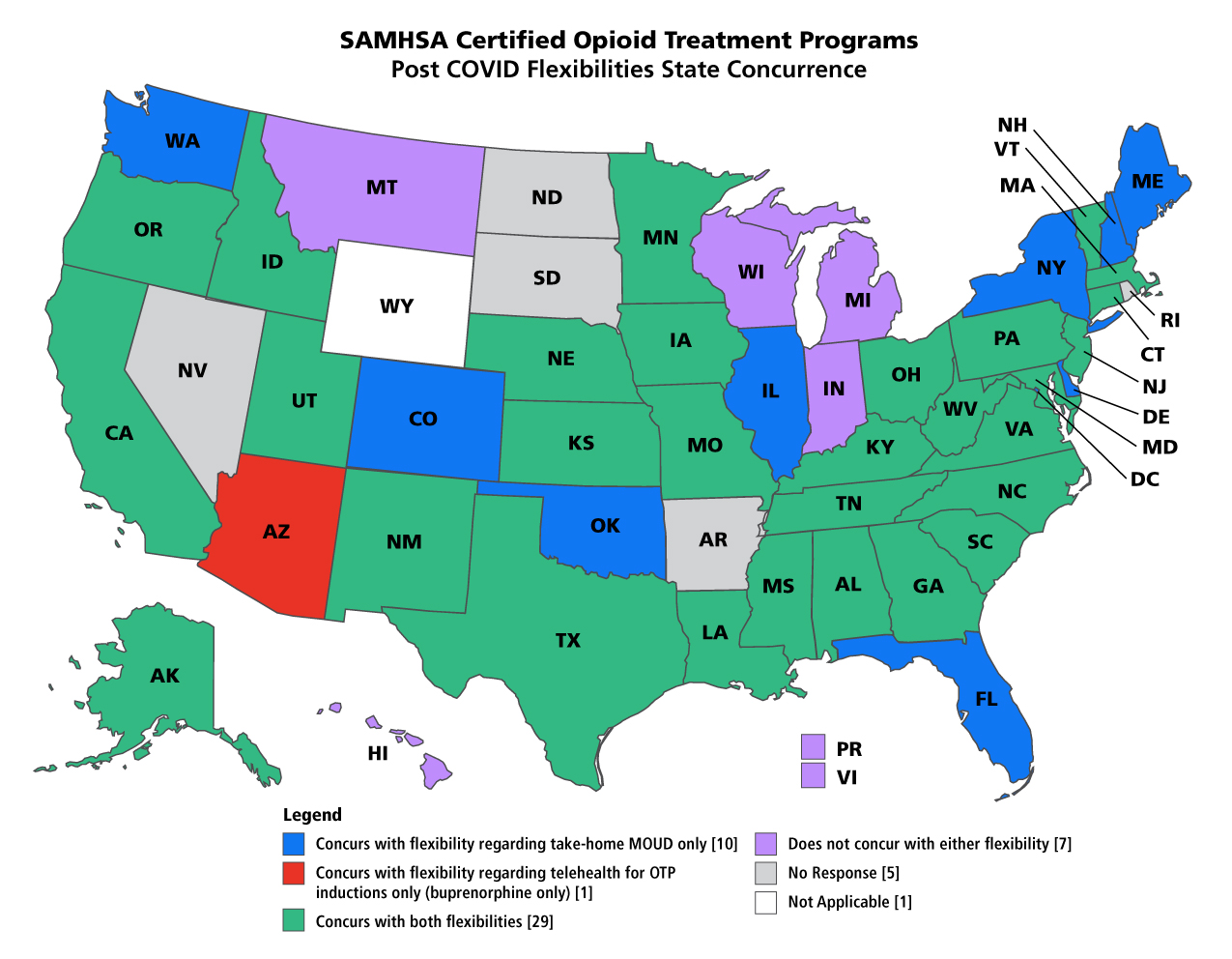
Read more at SAMHSA's Take-Home Methadone Extension Guidance webpage.
Methadone Take-Home Flexibilities For Patients Did Not Correlate With Increased Use of Illicit Drugs
Among Study of Patients At An OTP in Washington
In a pre- post-COVID exemption analysis, researchers at an OTP in Washington found evidence contrary to their initial hypothesis - that increases of unsupervised supply of methadone to patients would be associated with increased substance use. The analysis was conducted using a linear regression model and assessed the association between increased take-home doses and use of illicit opioids. In a secondary analysis of unadjusted descriptive data, when grouped by change in substance use, the clients who experienced a decrease in the use of morphine, codeine, and heroin post-COVID-19 were given significantly more take-home doses than the groups that had no change or an increase in these substances. The results of urine drug tests (UDTs) were 26.2% vs. 36.3% positive for 6-acetylmorphine, 32.6% vs. 40.6% positive for codeine, 34.2% vs 44.2% positive for hydromorphone, 39.5% vs. 48.1% positive for morphine, 8.0% vs. 14.4% positive for fentanyl (p-value < .001). Furthermore, in the adjusted model, there was no significant relationship between change in opioid use and increased receipt of take-home methadone doses. Ultimately, although take-home doses post-COVID-19 nearly doubled, this increase was not associated with a significant change in use of illicit opioids.
Panwala, Victoria, Emily Thorn, Solmaz Amiri, M. Eugenia Socias, Robert Lutz, and Ofer Amram. “Opioid Use and COVID-19: A Secondary Analysis of the Impact of Relaxation of Methadone Take-Home Dosing Guidelines on Use of Illicit Opioids.” The American Journal of Drug and Alcohol Abuse, July 11, 2023, 1–9.
Access here.
Providers' Experiences of Methadone Take-Home Flexibilities Were Broadly Positive
Blocking Evolution of Methadone Treatment for OUD Impediment to Patient-Centered Care
The pandemic shifted the perceived risks and benefits of daily clinic attendance and led to widespread changes allowing for observation of more flexible prescribing. In a qualitative systematic review of providers' experiences with relaxing restrictions on take-home doses of medications prescribed for OUD during the COVID-19 pandemic, authors found providers' experiences of the relaxation of these restrictions were broadly positive.
Three analytical themes emerged from providers: (1) providers were initially cautious of changes and after observing few to no negative consequences, came to support the flexibilities, (2) providers developed new processes to balance risks assume by clients, society, and providers, including greater use of team-based decision-making, and (3) providers found that structural reforms removed impediments to person-centered and individualized care, client autonomy, and facilitated provider-client relationships. The authors suggest that the evidence indicates that continuing to restrict methadone treatment for OUD through regulations and policies conflicts with person-centered approaches to care and that stronger guidance and support from regulatory agencies may help increase uptake of such flexibilities.
Adams, Alison, Sarin Blawatt, Scott MacDonald, Rhys Finnick, Julie Lajeunesse, Scott Harrison, David Byres, Martin T. Schechter, and Eugenia Oviedo-Joekes. “Provider Experiences with Relaxing Restrictions on Take-Home Medications for Opioid Use Disorder during the COVID-19 Pandemic: A Qualitative Systematic Review.” The International Journal on Drug Policy 117 (July 2023): 104058.
Access here.
Methadone Distribution for Pain Treatment Associated With Diversion and Overdose, 2002 to 2014
In a seminal study published in 2016, authors used a Joinpoint regression to examine trends and found the use of prescription opioid methadone for treatment of pain, rather than for treatment of OUD, as an important contributor to the rise in methadone-involved opioid-related overdose deaths. Their findings were that between 2002 and 2014, there was a strong positive association between trends in methadone distribution for use in pain treatment, and methadone diversion and methadone-involved overdose deaths.
The declines in methadone-involved overdose deaths coincided with actions aimed at reducing methadone use for pain, including, in 2006, the Food and Drug Administration (FDA)-issued warnings on the risks of prescribing methadone for pain and revised the dosing interval from every 3-4 hours to every 8-12 hours.
Jones, Christopher M., Grant T. Baldwin, Teresa Manocchio, Jessica O. White, and Karin A. Mack. “Trends in Methadone Distribution for Pain Treatment, Methadone Diversion, and Overdose Deaths — United States, 2002–2014.” Morbidity and Mortality Weekly Report 65, no. 26 (2016): 667–71.
Access here.
Prescribers Should Be Specialized and Follow Guidelines When Prescribing Methadone
An analysis of rates of fatal methadone overdoses and sales nationally during 1999-2010 and rates of overdose death for methadone, when compared with rates for other major opioids in 13 states in 2009, found methadone disproportionately contributed to the excessive number of opioid pain reliever (OPR) deaths. The authors suggest prescribers who choose to prescribe methadone should have substantial experience with its use and follow consensus guidelines.
Paulozzi, Leonard, Karen Mack, and Christopher M. Jones. “Vital Signs: Risk for Overdose from Methadone Used for Pain Relief — United States, 1999–2010,” July 6, 2012. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6126a5.htm.
Access here.
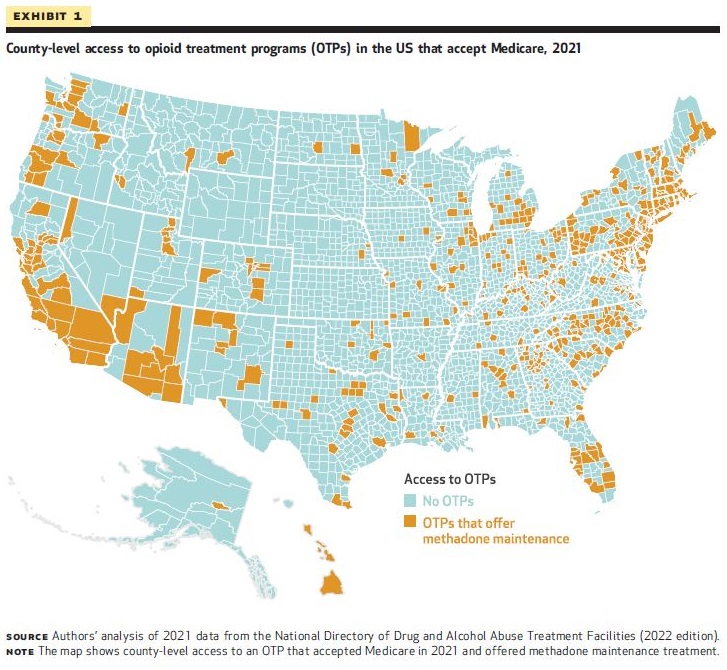
Large Gaps in Access to Methadone for Medicare Beneficiaries in Rural Midwest, South, and West
Methadone Dispensed in Pharmacies and FQHCs Could Significantly Improve Access
In 2020, Medicare began reimbursing for OTP services for the first time. Researchers examining 2021 data examining county-level factors associated with OTPs accepting Medicare found 16.3% of counties had at least 1 OTP that accepted Medicare. In 124 counties the OTP was the only specialty treatment facility offering any form of medication for OUD. The researchers' regression results showed that the odds of a county having an OTP that accepted Medicare were lower for counties with higher versus lower percentage of rural residents and lower for counties located in the Midwest, South, and West compared with the Northeast. Therefore, while the Medicare OTP benefit improved the overall availability of medications for OUD for Medicare beneficiaries, geographic gaps remain, and in 2021:
- Approximately 21.6 million Medicare beneficiaries lived in a county without an OTP that accepted Medicare,
- 10.4 million beneficiaries lived in a county without an OTP that accepted Medicare, and
- 10.4 million beneficiaries lived in a county without any access to MOUD in a specialty SUD treatment facility (OTP or non-OTP).
According to the authors, their findings indicate that allowing for methadone to be dispensed in pharmacies or federally qualified health centers (FQHCs) may help address geographic disparities in access to methadone.
Harris, Samantha J., Courtney R. Yarbrough, and Amanda J. Abraham. “Changes In County-Level Access To Medications For Opioid Use Disorder After Medicare Coverage Of Methadone Treatment Began.” Health Affairs 42, no. 7 (July 2023): 991–96.
Access Harris, et. al. study in Health Affairs here.
CMS Medicare Policies Were Important First Step in Increasing Access for Medicare Beneficiaries to Methadone Treatment for OUD
In a cross-sectional study of methadone and buprenorphine dispensing by beneficiary characteristic of the impacts of the Centers for Medicare and Medicaid Services (CMS) instituting a bundled payment reimbursement policy for OUD treatment and policies implemented by the Substance Abuse and Mental Health Administration and CMS Medicare designed to facilitate access to treatment for OUD during the COVID-19 pandemic, researchers found methadone dispensing increased among Medicare beneficiaries after the policy changes in January 2020. Their findings suggest a strong uptake by beneficiaries younger than 65 years largely driven by dually eligible beneficiaries. However, the rate of increase slowed or even reversed in the most recent quarter for all groups (in January 2022). The rates of buprenorphine dispensing did not provide evidence beneficiaries substituted buprenorphine for methadone.
Taylor EA, Cantor JH, Bradford AC, Simon K, Stein BD. Trends in Methadone Dispensing for Opioid Use Disorder After Medicare Payment Policy Changes. JAMA Netw Open. 2023 May 1;6(5):e2314328. doi: 10.1001/jamanetworkopen.2023.14328. PMID: 37204793; PMCID: PMC10199341.
Access here.
A Critical Examination of Two Opportunistically-Used Studies
Policymakers must find an appropriate balance between weighing the risks and benefits of public health interventions.
This may be no where more apparent than it is with treatment with methadone for opioid use disorder. Decisions in front of policymakers have been made tougher by two widely-circulated studies, which raise questions about a potential association between the methadone flexibilities instituted during COVID-19 and the methadone-involved overdose rate in 2020. Below, highlights are included to help with your critical examination of these studies.
Robert A. Kleinman, Marcos Sanches, Methadone-involved overdose deaths in the United States before and during the COVID-19 pandemic, Drug and Alcohol Dependence, Volume 242, 2023, 109703, ISSN 0376-8716.
This study finds an increase in methadone-involved overdose deaths in the year after March 2020 compared with prior trends, both with and without co-involvement of synthetic opioids, and large relative increases in methadone-involved deaths among Hispanic and non-Hispanic Black individuals, which prompts the authors to warn against permanently relaxing take-home methadone regulations without further study.
Highlights for critical examination:
- In their year-over-year analysis, the authors do not control for the step increase in mortality level that occurred in the early spring of 2020, and assume it could be attributed to the change in methadone regulations. However, the increase in mortality level also has been found in overdose deaths that did not involve methadone, and in related domains, such as alcohol use disorder deaths.
- The authors examine absolute counts, rather than relative rate increases, in methadone-involved overdose deaths. (Relative rates are in proportion to the whole, while absolute counts are not.) For example, the relative rate of methadone-involved overdose deaths declined 9.5% between August 2021 and 2022 (for reference, see "letter from addiction clinicians," above).
- The authors do not include or examine additional, provisional overdose death data available from after March 2021, even though it was available, when the rate of methadone-involved overdose deaths stabilized and declined. This may reflect bias in the model in the study.
- The authors state “[w]e hope that these findings will not add to further misconceptions about the safety of methadone relative to other less widely prescribed Schedule II opioids.”
Daniel E. Kaufman, Amy L. Kennalley, Kenneth L. McCall, Brian J. Piper, Examination of methadone involved overdoses during the COVID-19 pandemic, Forensic Science International, Volume 344, 2023, 111579, ISSN 0379-0738.
This study examines methadone overdoses 1999 to 2020 and finds an increase of methadone-involved overdose deaths by 48.1% in 2020 relative to 2019, which prompts authors to suggest further study of flexibilities for methadone take-homes before they are made permanent.
Highlights for critical examination:
- The authors do not include or examine additional, provisional overdose death data after March 2021, even though it was available to them, when the rate of methadone-involved overdose deaths stabilized and declined, which may reflect bias the model in the study.
- The authors point out that the rate of methadone-involved overdose deaths in 2020 was much lower than its peak in 2006-2008, and that such deaths were largely attributed to methadone prescribed for treating pain.
- The authors state that their study is "observational and does not allow for a causal attribution of the increase in methadone-involved overdose deaths to any specific factor,” and that the study "cannot distinguish whether individuals who die from methadone-involved overdoses receive methadone through OTPs, as prescriptions for pain, or through other sources, including diverted methadone.”